Kristin Bowman-James

- Daryle Busch Distinguished Professor of Inorganic Chemistry
- Director Kansas NSF EPSCoR
Contact Info
Lawrence
1567 Irving Hill Rd
Lawrence, KS 66045
Education —
1974-1975
Specialization
Supramolecular and Traditional Coordination Chemistry
Research —
Bowman-James Research Group Poster
Research in the Bowman-James group involves the strategic design of organized molecular frameworks as selective receptors for anions as well as potential ligands for transition metal ions. Our interest in these areas stems from potential environmental and biological applications in sensing, separations, catalysis, and biomimetic chemistry. We also seek to examine the similarities (and differences) between supramolecular anion coordination chemistry and transition metal coordination chemistry.
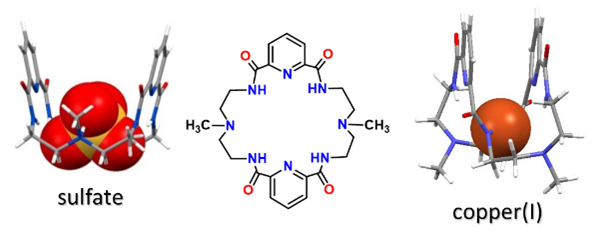
Hence, we frequently explore the same ligands as frameworks for coordinating both anions and transition metals as shown in the figure for one of our amide-based macrocyclic ligands. One basic similarity between hydrogen bond and transition metal coordination is the donation of an electron pair. For anion coordination the electrons are donated from the anion to the ligand, while in transition metal chemistry, the donation is from the ligand to the metal ion (see ChemDraw diagram).
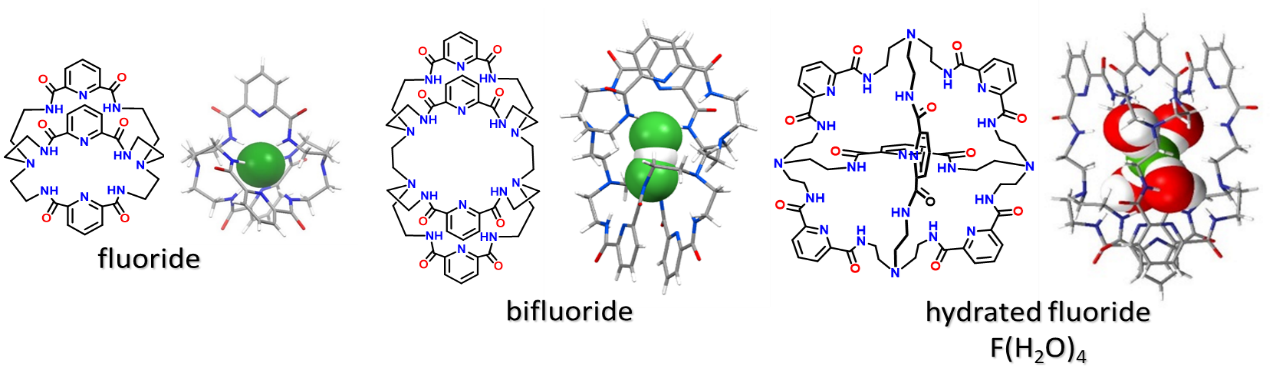
Examples of different fluoride-containing complexes illustrate the wide range of anion binding ligands that we designed.
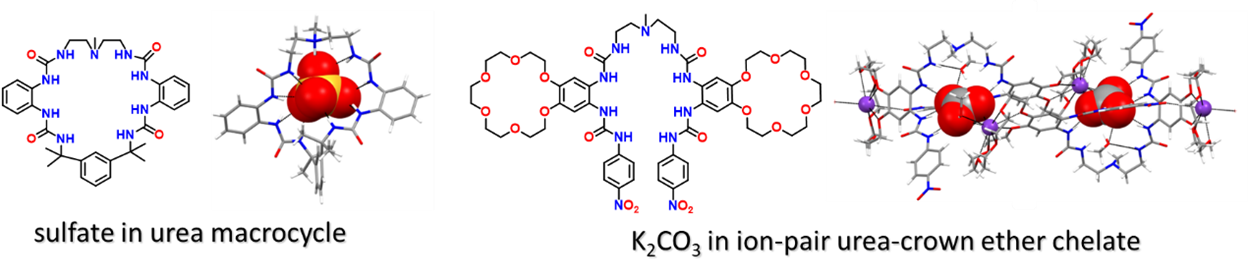
Our research especially targets anions of environmental relevance. We focus on current day challenges including remediation, such as nuclear waste site clean-up, as well as ways to address the looming depletion of the world’s available phosphorus reserves. As a result, we have explored the capture of small oxo anions, halides, and larger carboxylates and polyphosphates. Anions of interest range in shape, from monoatomic to complex polyatomic anions; and charge, from -1 to -12. One goal has been to design anion ligands capable of binding sulfate for nuclear remediation efforts at waste sites such as the National Laboratory at Hanford, in Washington state. While seemingly innocuous, sulfate’s physical properties, e.g., lack of solubility in borosilicate glass and caustic nature, are detrimental to separations efforts. Newer hosts include a urea macrocycle that binds sulfate selectively and a new ion pair host design as shown below.
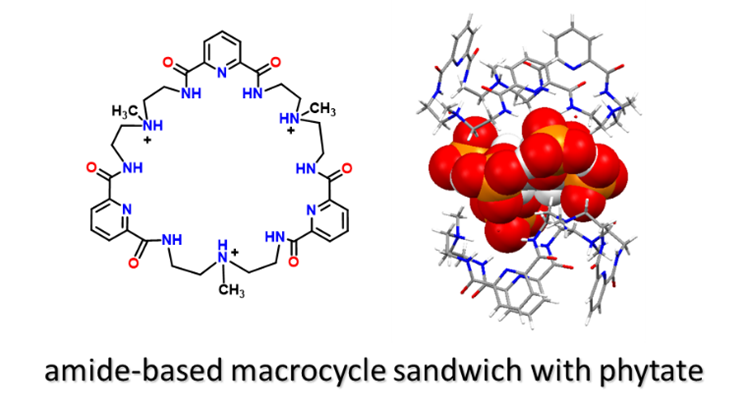
A newer project, targeting growing concern about world-wide phosphorus reserves, involves phosphorus anions, particularly those of agricultural and environmental relevance. Phosphorus plays crucial roles in the well-being and growth of plants, with significant impacts on global food sources. We are using some of our macrocyclic hosts to bind phytate, an inositol hexaphosphate anion that is prominent in soils and in plant metabolism, but for which not much structural information is known. Since this polyphosphate anion contains almost 30% phosphorus by weight, and is the most prevalent organophosphate in soils, it could be a significant source of readily available phosphorus. This interesting anion is known as an anti-nutrient because of its affinity for metal ion nutrients, rendering them insoluble and ineffective. It also can possess an incredible maximum -12 charge for such a small anion. We have succeeded in obtaining important structural information by using our macrocyclic hosts to sequester phytate followed by elucidating their crystalline structures using single crystal X-ray crystallographic techniques. The structure is shown below, and is only one of a number of structures we have now obtained, providing significant new information about this intriguing anion.
Selected Publications —
J. Lohrman, S. Pramanik, S. Kaur, H. Telikepalli, V. W. Day, K. Bowman-James, Hydrophilic and hydrophobic carboxamide pincers as anion hosts, Org. Biomol. Chem. 2021, 8516-8520. DOI: https://doi.org/10.1039/D1OB01605A
Q.-Q. Wang, V. W. Day, K. Bowman-James, Chemistry and Structure of a Host Guest Relationship: The Power of NMR and X-ray Diffraction in Tandem, J. Am. Chem. Soc. 2013, 135(1), 392-399. DOI: 10.1021/ja3096762
B. A. Moyer, R. Custelcean, B. P. Hay, J. L. Sessler, K. Bowman-James, V. W. Day, S.-O. Kang, A Case for Molecular Recognition in Nuclear Separations: Sulfate Separation from Nuclear Wastes, Inorg. Chem., 2013, 52(7), 3473-3490. DOI: 10.1021/ic3016832
Q.-Q. Wang, V.W. Day, K. Bowman-James, Hexagonal Molecular “Palladawheel,” Chem. Commun. 2013, 49(73), 8042-8044. DOI: 10.1039/C3CC44714F
Q.-Q. Wang, R. A. Begum, V.W. Day, K. Bowman-James, Chemical Mustard Containment Using Simple Palladium Pincer Complexes: The Influence of Molecular Walls, J. Am. Chem. Soc. 2013, 135(45), 17193-17199. DOI: 10.1021/ja408770u
Q.Q. Wang, V. W. D.; K. Bowman-James, Macrocyclic Influences in CO2 Uptake and Stabilization, Org. Lett 2014, 16, 3982-3985. DOI: 10.1021/ol501812u
C. Jia, Q.-Q, Wang, R.A. Begum, V. W. Day, and K. Bowman-James, Chelate effects in sulfate binding by amide/urea-based ligands, Org. & Biomolec. Chem. 2015, 13(15), 6953-6957. Selected for cover art. DOI: 10.1039/C5OB00618J
J. Lohrman, H. Telikepalli, T. S. Johnson, T. A. Jackson, V. W. Day, and K. Bowman-James, Pyrazinetetracarboxamide: A Duplex Ligand for Palladium(II), Inorg. Chem. 2016, 55 (11), 5098-5100. DOI: 10.1021/acs.inorgchem.6b00594
S.-O. Kang, T. S. Johnson, V. W. Day, and K. Bowman-James, Pyridine-2,6-dicarboxamide pincer-based macrocycle: a versatile ligand for oxoanions, oxometallates, and transition metals, J. Supramolec. Chem. 2018, 30(4), 305-314. DOI: 10.1080/10610278.2017.1361534.
J. Lohrman, E. A. Vázquez-Montelongo, S. Pramanik, V. W. Day, M. A. Hix, K. Bowman-James, G. A. Cisneros, Characterizing Hydrogen-Bond Interactions in Pyrazinetetracarboxamide Complexes: Insights from Experimental and Quantum Topological Analyses, Inorg. Chem. 2018, 57, 9775-9778. DOI: 10.1021/acs.inorgchem.8b00627
M. Reinmuth, S. Pramanik, J. T. Douglas, V. W. Day, K. Bowman-James, Structural Impact of Chelation on Phytate, a Highly Phosphorylated Biomolecule, Eur J. Inorg. Chem. 2019, (Iss. 14), 1870-1874. DOI: 10.1002/ejic.201900091, Phosphorus 2019 category. Invited for cover page and cover profile.
K. Bowman-James, Supramolecular cages trap pesky anions, Science 2019, 365, 124-125.
S. Pramanik, V. W. Day, K. Bowman-James, Supramolecular traps for highly phosphorylated inositol sources of phosphorus, Chem. Commun. 2020, 56, 3269-3272. DOI: 10.1039/c9cc09321d
S. Kaur, V. W. Day, K. Bowman-James, Urea-Based Macrocycle Selective for Sulfate and Structurally Sensitive to Water, Cryst. Growth & Des. 2020, 20, 4212-4216. DOI: 10.1021/acs.cgd.0c00411
S. Kaur, S. Pramanik, V. W. Day, K. Bowman-James, Snapshots of “crystalline” salt-water solutions of inositol hexaphosphate conformers, Dalton Trans. 2021, 50, 480-484. DOI: 10.1039/d0dt03775c
S. Kaur, P. Kumarj R. Gupta, K. Bowman-James, Pincers based on dicarboxamide and dithiocarboxamide functional groups in Pincer Compounds, 2018, 295-325.
Supramolecular Chemistry of Anions, A. Bianchi, K. Bowman-James, E. García-España, Eds. Wiley-VCH: New York, 1997, 461 pp.
Adv. Inorg. Chem., From the Template Effect to Spontaneous Intermolecular Organization, A Celebration of Daryle Busch's First 50 years of Leadership Teaching, Research and Service. R. van Eldik and K. Bowman-James, Eds. (Proceedings held in Philadelphia, Pennsylvania, 2004.) 2007, 59, 320 pp.
Anion Coordination Chemistry, K. Bowman-James, A. Bianchi, E. García-España, Eds., Wiley-Interscience, 2012, 560 pp.
Awards & Honors —
American Academy of Arts and Sciences Fellow
2021
American Chemical Society Award in Inorganic Chemistry
2021
University of Kansas Leading Light Award
2011
American Chemical Society Fellow
2010
University Distinguished Professor
2007
Temple University Gallery of Success
2004
St. Louis Section Midwest Award of the American Chemical Society
2003
University of Kansas Higuchi Award - Dolph Simons Award for Research in the Biomedical Sciences
2002
ACS Women Chemists Committee Regional Award for Diversity
2002
Iota Sigma Pi Award for Professional Excellence
2002